Medicine moves quickly when it has to. In my previous post I mentioned clinical trials of DOACs for patients with COVID-19, but we already know that this approach is proving to be futile. We’ve seen the spectre of blood clots appear as a side effect from the vaccine, and we now think we know how they occur.
Clearly the risk of vaccine-induced immune thrombotic thrombocytopenia at a population level is outweighed by the benefit of the vaccine; equally the DOACs, while excellent in many ways, do not provide the wholly safe anticoagulation that we have been hoping for. But can we predict what might lay over the horizon in the field?
Before we look at what the future might hold, however, a little history lesson.
The design, development and clinical approval of the DOACs is a success story for rational drug development, despite the path to their clinical application being at times a rocky one. The elucidation and sequencing of the coagulation cascade went hand-in-hand with a hit-and-miss approach to treatment and prevention of clots. It was often based on serendipitously observed action of naturally occurring compounds that acted on multiple targets. As a result, though, we now understand how blood clots form in normal circumstances.
In brief, when damage to blood vessels occurs, tissue factor (TF) on the surface of cells outside the vessel binds to circulating activated factor VII (FVIIa). The TF-FVIIa complex then activates more FVII, creating more of itself, which in turn activates small amounts of factors IX and X. Activated factor X (FXa) binds to its cofactor, activated factor V (FVa), and the resulting prothrombinase complex generates thrombin. This is the ‘initiation phase’.
Thrombin is central to clotting. It is the enzyme that cleaves fibrinogen to form fibrin – the protein that constitutes the fibres in a clot. It also activates platelets and leads to the activation of other clotting factors, notably von Willebrand Factor (vWF), FV, FVIII, FIX and FXI, in the ‘amplification phase’. And with these players in place, the scene is now set for large-scale thrombin generation.
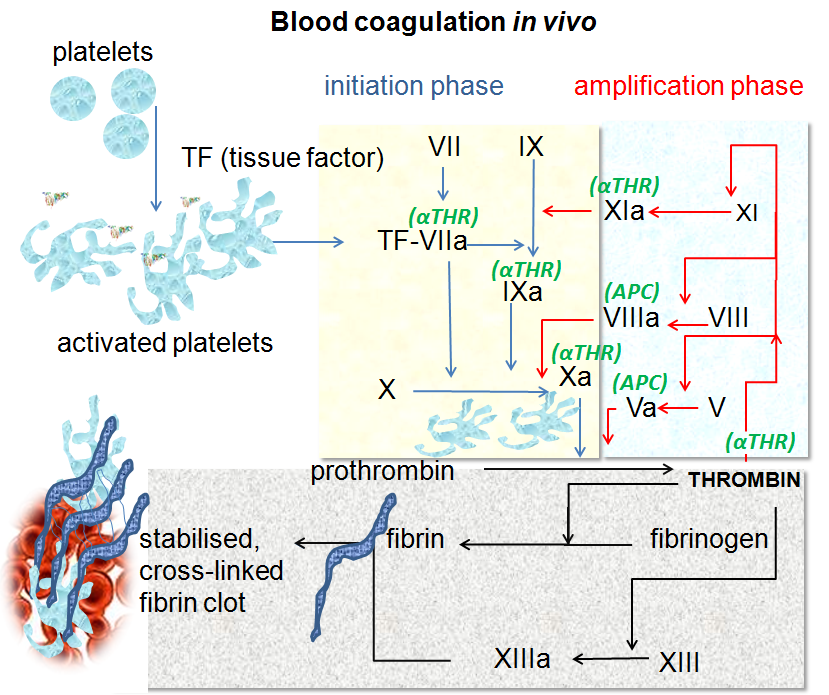
In the final step of the cascade, or ‘propagation phase’, FVIIIa and FIXa form the ‘tenase’ complex on the surface of activated platelets. Tenase rapidly generates FXa, in turn activating yet more thrombin – the ‘thrombin burst’. This creates more fibrin and activates more platelets, and the clot grows.
While thrombin is clearly the sharp end of the clotting cascade, being directly responsible both for platelet activation and fibrin fibre formation, its activation depends on FXa. This makes FXa an attractive target for controlling inappropriate clotting, as we might predict that fewer inhibitor molecules would be required to sufficiently reduce FXa levels. But we would also expect some thrombin to remain active, and might therefore imagine bleeding side effects to be more manageable than if we had completely inhibited thrombin itself.
Factor Xa seems like an obvious druggable target, but it wasn’t until 1987 that the first natural FXa inhibitor, antistasin, was isolated – from the Mexican leech. Then in 1990 the tick anticoagulant peptide (TAP) was isolated from the soft tick Ornithodoros moubata. TAP and antistasin are tight-binding inhibitors of FXa, and were used to validate FXa as a viable drug target. Although these two compounds were not further developed, they did pave the way for the development and eventual clinical approval of three FXa-inhibiting DOACs: apixaban, edoxaban and rivaroxaban.
These molecules have several advantages over other anticoagulants – they don’t require regular monitoring because they have predictable pharmacodynamics and pharmacokinetics, they have reasonably straightforward dosing adjustments to account for kidney function, body weight or age, they are oral tablets, and they are associated with less of the more severe types of bleeding than vitamin K antagonists such as warfarin.
Nonetheless, we know the DOACs are not perfect. Although elimination of the FXa inhibitors from the body does not occur primarily through the kidneys, dose reductions are required for people with chronic kidney disease, and they are not recommended for people with end-stage renal disease. And as we’ve seen, bleeding with DOACs is still a concern in a few indications and for many physicians. Off-label dose reduction does not appear to work to reduce bleeding, and furthermore tends to expose the patient to an increased risk of clotting. As ischaemic strokes are usually far more serious than bleeding events, inappropriate dose reduction of DOACs is not a responsible strategy – as well as being off-label!
The question is, then, will we ever be able to design a ‘safe’ anticoagulant? Or one that we can give to people requiring dialysis for failing kidneys? As it turns out, scientists at several pharmaceutical companies clearly think so. And the target for their interest is clotting factor XI.
Factor XI activates FIX in the amplification phase of blood clotting, leading to the formation of the tenase complex. It can also activate factors V, VIII, X and XII, and promotes clot stability by inhibiting coagulation regulators. In addition, FXIa contributes to platelet activation.
Spontaneous bleeding in people with congenital FXI deficiency (haemophilia C) is generally rare, and less severe than in those with the more familiar haemophilia A or B (FVIII and FIX deficiency, respectively). Patients with severe FXI deficiency tend to have a reduced risk of thrombotic disorders such as deep vein thrombosis (DVT) and ischaemic stroke, while increased levels of FXI are associated with increased risk of DVT and stroke, as well as myocardial infarction and cardiovascular disease.
As a result, in recent years there has been intense patent activity surrounding FXI and its inhibition. Compounds that have been tested for activity against FXI – and therefore for the treatment and prevention of thrombosis – in preclinical and clinical studies include monoclonal antibodies, small molecules (both oral and parenteral agents), polypeptides, DNA and RNA aptamers, and antisense mRNA oligonucleotides.
Although it’s still early days, first signs are encouraging. There are currently no worrying bleeding signals (although numbers of participants have been small in these trials), and some of the agents are showing promise in reducing thrombosis in patients on dialysis for chronic kidney disease.
Here at AS&K we have been interested in and working with clients in cardiovascular disease for more than a decade. Although the field might not move as quickly as vaccine development during a pandemic, it’s still an exciting area, with much more to come. We’re looking forward to what will happen in the field over the next 10 years!
Image source: By Dr Graham Beards - Own work (Based on Figure 7.5 on page 167 of "Blood Science: principles and pathology"by Andrew Blann and Nessar Ahmed, Publishers Wiley Blackwell (2014) ISBN:978-1-118-35146-8), CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=19772662